SAHPRA’s digital transformation is a priority for the organisation as our focus is on building capabilities and systems that will allow the institution to be more agile, innovative, streamlined, and efficient in the delivery of its mandate.
At the centre of this digitisation will be intuitive self -service customer portals for the end-user, as well as intelligent internal management and review systems to streamline the entire regulatory process. Underpinned by a data analytics tool the enables real-time reporting and insights.
The ICT Strategic Roadmap
The Information Technology Strategic Plan sets out a five-year strategic direction for Information and Communication Technology (ICT) in response to SAHPRA’s key strategic objectives which include the strengthening of Information and Communication Technology and the Digital Transformation of SAHPRA’s business processes.
The 5 ICT Strategic Principles
Principle 1
Principle 2
Principle 3
Principle 4
Principle 5
Deliver Value enabled by appropriate ICT Governance practices and frameworks – Strong and effective corporate governance of ICT will cultivate a culture of strategic ownership and control over IT direction and investment, thereby ensuring that there is alignment between IT priorities and expenditure and the organization.
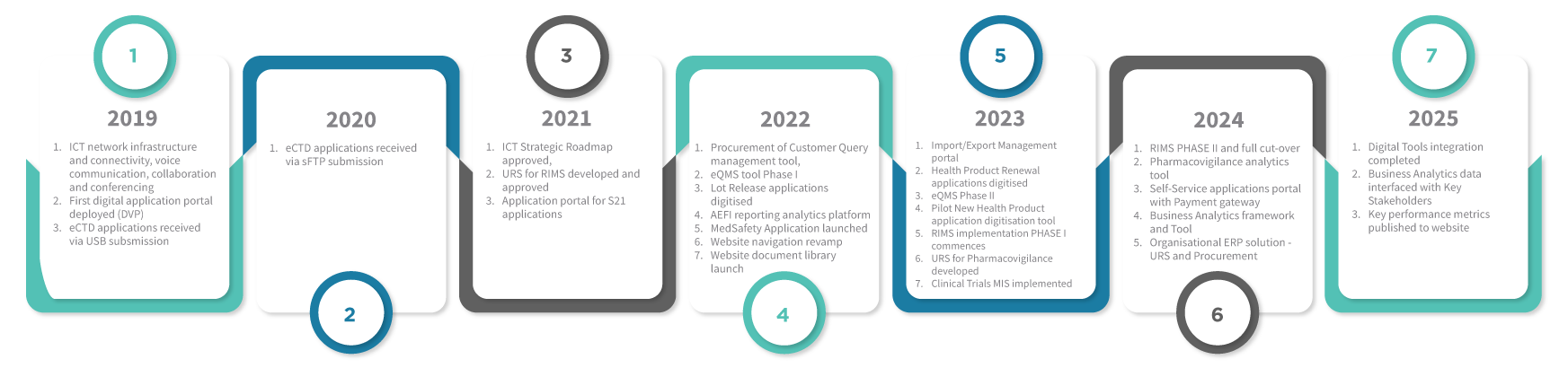
Digitisation Roadmap
Comments from the COO
One of the Strategic Levers for the organization is to successfully digitilise our business processes and create efficiencies that will enable the organisation to drive improved service delivery, improved turn-around times and improved access to real-time information. This will enable sound decision making, keeping our various stakeholder informed and rapidly provide pertinent information when and where required. The Information Communications and Technology unit has been working closely with our internal stakeholders to improve the digital execution maturity of the organization, to capture the user requirements specifications and identify suitable digital solutions to the needs of the organisation. We have managed to establish sound network and connectivity infrastructure, collaborations and communications platforms and will be focusing immense effort and resource in 2023 and 2024 periods to implement a number of software tools for core operational processes and will be engaging our industry stakeholders during the implementation processes to keep you informed, and ensure we have training sessions available to enable our external users to use the tools optimally in order to derive maximum benefit for all parties involved.
Milestones achieved so far!
Coming Soon!
The To-Do List!
Pharmacovigilance Signal Detection and Case Management platform
Market research and User Requirement Specification (URS) compilation to be completed
Batch Tracking Tool
This is linked to Health Product Master data management and will allow health product consumers to verify authenticity of products and lots available in authorised health product supply points.
Latest News
- Renewals applications Live – 1 August 2023
Upcoming events
Coming soon.