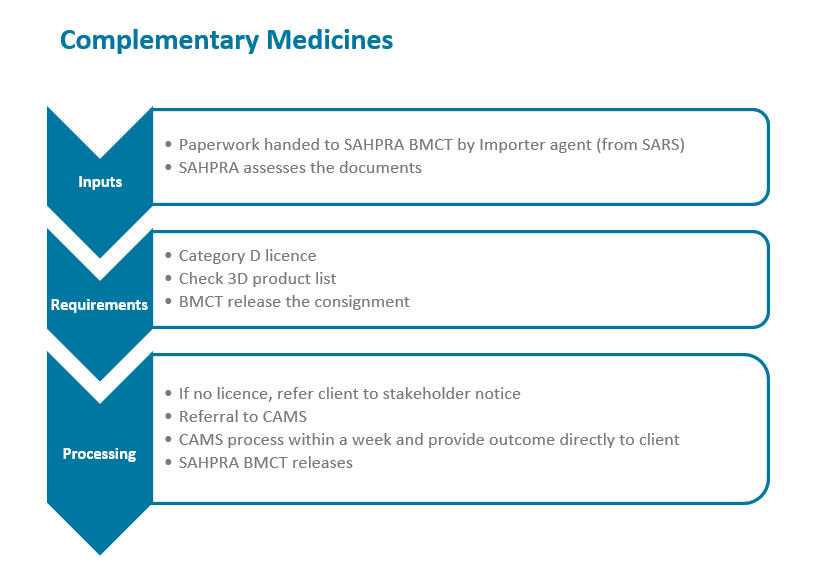
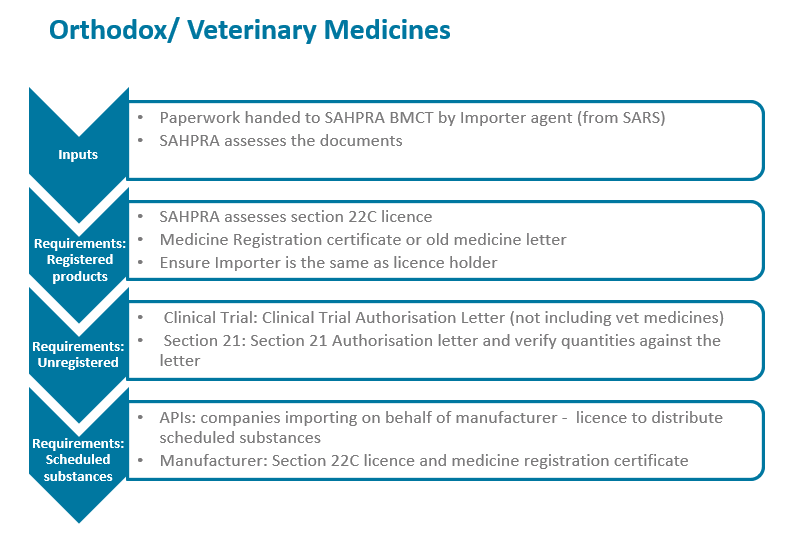
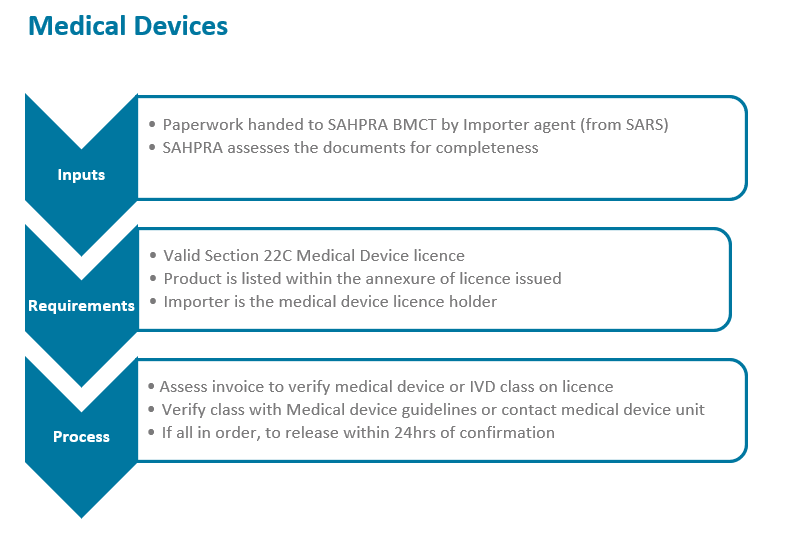
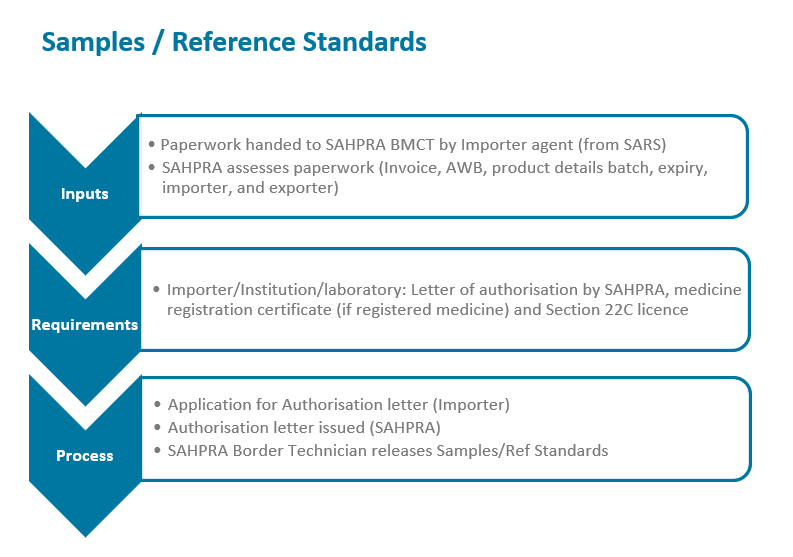
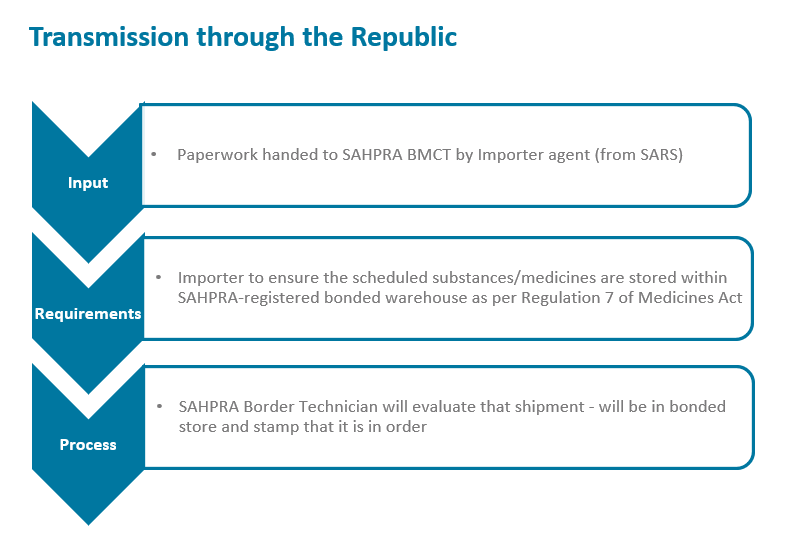
The South African Health Products Regulatory Authority (SAHPRA) has a process flow for the importation of medical products into the Republic of South Africa in terms of the Medicines and Related Substances Act 101 of 1965, as amended. Please see specific process flows below for:
- Complementary Medicines
- Orthodox / Veterinary Medicines
- Medical Devices
- Sample / Reference Standards
- Transmission through the Republic